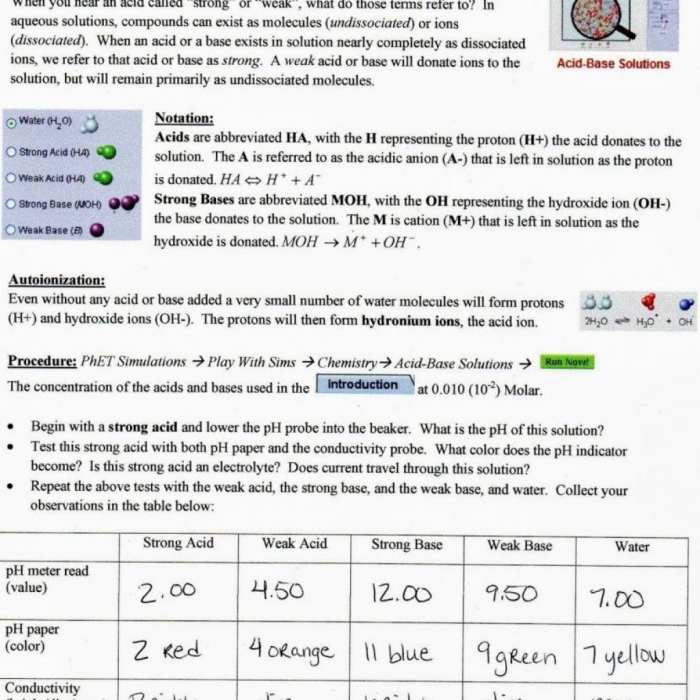
Isotopes and atomic mass phet answer key – Welcome to the captivating world of isotopes and atomic mass, where the Phet simulation and answer key serve as your guide to unraveling the mysteries of chemistry. Delve into the significance of isotopes in understanding atomic structure and nuclear reactions, while exploring the practical applications that shape our understanding of medicine, environmental science, and archaeology.
As we embark on this scientific journey, we will delve into the concept of atomic mass, examining its role in determining the properties of elements and the methods used to measure it. Through the Phet simulation, you will witness the dynamic interplay of isotopes and atomic mass, gaining hands-on experience that will illuminate these complex concepts.
Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron number results in different atomic masses for the isotopes. Isotopes are significant in chemistry because they can affect the chemical properties of an element.
For example, the isotope carbon-12 is used as the standard for atomic mass, while the isotope carbon-14 is used in radiocarbon dating.
Examples of Isotopes and Their Uses, Isotopes and atomic mass phet answer key
There are many examples of isotopes and their uses in various fields. Here are a few examples:
- Carbon-12is the most common isotope of carbon and is used as the standard for atomic mass.
- Carbon-14is a radioactive isotope of carbon that is used in radiocarbon dating to determine the age of organic materials.
- Uranium-235is a radioactive isotope of uranium that is used in nuclear reactors to produce energy.
- Iodine-131is a radioactive isotope of iodine that is used in medical imaging to diagnose and treat thyroid disorders.
Role of Isotopes in Understanding Atomic Structure and Nuclear Reactions
Isotopes play an important role in understanding atomic structure and nuclear reactions. The number of protons in an atom determines the element, while the number of neutrons determines the isotope. The atomic mass of an element is the weighted average of the masses of its isotopes.
Isotopes can be used to study nuclear reactions because they can be used to track the movement of atoms and to identify the products of nuclear reactions.
Atomic Mass
Atomic mass is the mass of an atom, expressed in atomic mass units (amu). The atomic mass of an element is the weighted average of the masses of its isotopes. Atomic mass is important in determining the properties of elements.
For example, the atomic mass of an element can be used to calculate its density and its melting point.
Methods Used to Measure Atomic Mass
There are several methods that can be used to measure atomic mass. One common method is mass spectrometry. Mass spectrometry involves separating atoms by their mass-to-charge ratio. Another method that can be used to measure atomic mass is nuclear magnetic resonance (NMR) spectroscopy.
NMR spectroscopy involves using a magnetic field to align the nuclei of atoms and then measuring the frequency of the radio waves that are absorbed by the nuclei.
Factors That Affect Atomic Mass
The atomic mass of an element is affected by several factors, including the number of protons, neutrons, and electrons in the atom. The number of protons and neutrons in an atom determines its mass number. The number of electrons in an atom does not affect its mass number, but it does affect its atomic mass.
Phet Simulation: Isotopes And Atomic Mass Phet Answer Key
The Phet simulation “Isotopes and Atomic Mass” is a tool that can be used to explore the concepts of isotopes and atomic mass. The simulation allows users to create and manipulate atoms and to measure their mass and charge. The simulation can be used to demonstrate the different types of isotopes and to show how the atomic mass of an element is affected by the number of protons, neutrons, and electrons in the atom.
How to Use the Simulation Effectively for Educational Purposes
The Phet simulation “Isotopes and Atomic Mass” can be used effectively for educational purposes in a variety of ways. The simulation can be used to:
- Demonstrate the different types of isotopes.
- Show how the atomic mass of an element is affected by the number of protons, neutrons, and electrons in the atom.
- Help students to understand the concepts of isotopes and atomic mass.
Answer Key
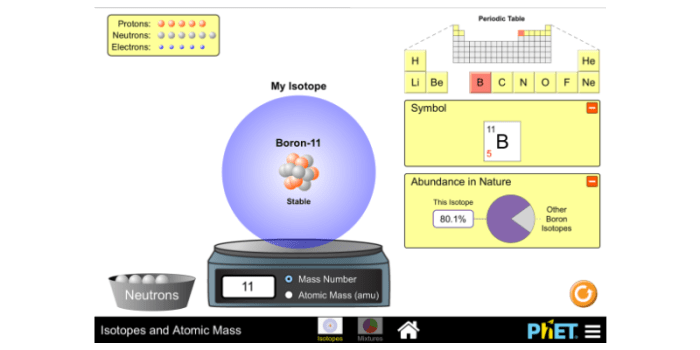
The answer key for the Phet simulation “Isotopes and Atomic Mass” is provided below. The answer key includes explanations for each question.
Section 1: Isotopes
- What are isotopes?
- How do isotopes differ from each other?
- What are some examples of isotopes?
- What are some uses of isotopes?
Section 2: Atomic Mass
- What is atomic mass?
- How is atomic mass measured?
- What factors affect atomic mass?
- How is atomic mass used to determine the properties of elements?
Applications
Understanding isotopes and atomic mass has practical applications in fields such as medicine, environmental science, and archaeology. In medicine, isotopes are used to diagnose and treat diseases. For example, the isotope iodine-131 is used to treat thyroid disorders. In environmental science, isotopes are used to study the movement of pollutants and to track the sources of pollution.
For example, the isotope lead-210 is used to track the movement of lead in the environment. In archaeology, isotopes are used to date artifacts and to determine the origin of materials. For example, the isotope carbon-14 is used to date organic materials.
FAQ Corner
What are isotopes?
Isotopes are variations of an element with the same number of protons but different numbers of neutrons.
How is atomic mass measured?
Atomic mass is measured using techniques such as mass spectrometry and nuclear magnetic resonance.
What is the Phet simulation “Isotopes and Atomic Mass” used for?
The Phet simulation is an interactive tool that allows students to explore the concepts of isotopes and atomic mass.